Getting Started | Common Questions
General contact and information:
- irb@vt.edu
- Phone:
540-231-3732
Federalwide Assurance:
FWA00000572
Institutional Official:
Daniel Sui, senior vice president, Research and Innovation
Institutional Review Board (IRB) Organization Number:
IORG0000389
IRB #1 (Regular IRB) Registration Number:
IRB00000667
IRB #2 (Emergency IRB) Registration Number:
IRB00012524
For a list of frequently used terms, see Definitions.
- For general questions, email irb@vt.edu or call 540-231-3732.
- For questions about a specific protocol, email the protocol coordinator (located on the summary page of the protocol management system). If unable to locate this information, email irb@vt.edu.
- Virtual office hours every Wednesday, 9 a.m. - 12 p.m. See the home page for more details and Zoom information.
- If interested in scheduling an individual consultation or classroom presentation, email irb@vt.edu.
All personnel listed on Virginia Tech protocols will need to complete the Collaborative Institutional Training Initiative (CITI) Social and Behavioral or Biomedical Basic course.
The following courses DO NOT meet the Human Rights Protection Program training requirement:
- Responsible Conduct of Research course of any kind
- Conflict of Interest
- Social and Behavioral or Biomedical Refresher courses (without previously completing a Basic course)
- Good Clinical Practice courses
For additional information, visit the training page.
Email irb@vt.edu if unsure of which training to complete, or who may need training.
All submissions are submitted through the protocol management system.
For questions about submitting a protocol to the Human Research Protection program, email irb@vt.edu.
Training
Researchers can meet Virginia Tech human subjects research training requirements through the Collaborative Institutional Training Initiative program (CITI). If the researcher has completed CITI training at another university, portions of that training may transfer. When logging into the CITI account, the researcher will need to affiliate with Virginia Tech and sign up for the appropriate courses. Any current modules required by both Virginia Tech and the previous institution should automatically populate, and those modules will not need to be retaken.
See Collaborative Institutional Training Initiative for additional information.
Research or funding from another institution
- For questions related to human subjects research or to schedule a consultation, contact irb@vt.edu.
- For questions related to funding, contact the Office of Sponsored Programs at osp@vt.edu.
- For questions related to research data, data security, or data transfers/agreements, contact the Privacy and Research Data Protection program at prdp@vt.edu.
All research projects are submitted through the protocol management system for review.
Human Subjects Research Determination
This submission is used to determine if the study will need review through the human subjects office. The information provided during the process aids in determining whether the activity meets the regulatory definition of research involving human subjects. Once the review is completed, the researcher will be given the appropriate steps to follow. All requests must include sufficient description of the activity and any supporting documents.
Interim Approval: This submission is typically used if funding is being sought and the funding source requires IRB documentation before releasing funds. Interim approval authorizes activities such as, purchasing of equipment, hiring study personnel, etc. This is NOT an IRB approval. It does not authorize the study team to commence research activities that involve human subjects. Following the interim, researchers will be asked to complete a full protocol to be reviewed through our office.
New Protocol
This is the full research protocol used when activities meet the definition of “Human Subjects Research” (HSR) in the Federal Regulations (45 CFR 46). When a full protocol is submitted a protocol coordinator will review the protocol, request any needed revisions, and make decisions about the appropriate review category for the study.
- Existing Data Protocol is used when the activities are deemed to be Human Subjects Research (HSR) and involves the use of an existing dataset that is identifiable or the identity of the participants can be ascertained by linking to other data sources.
- If researchers are unsure if this protocol would be appropriate, contact irb@vt.edu.
Submission Documents
Documents used in the submission process can be located on the Resources for Human Subjects Research page.
The type of review is determined by Human Research Protection program staff upon submission of a protocol.
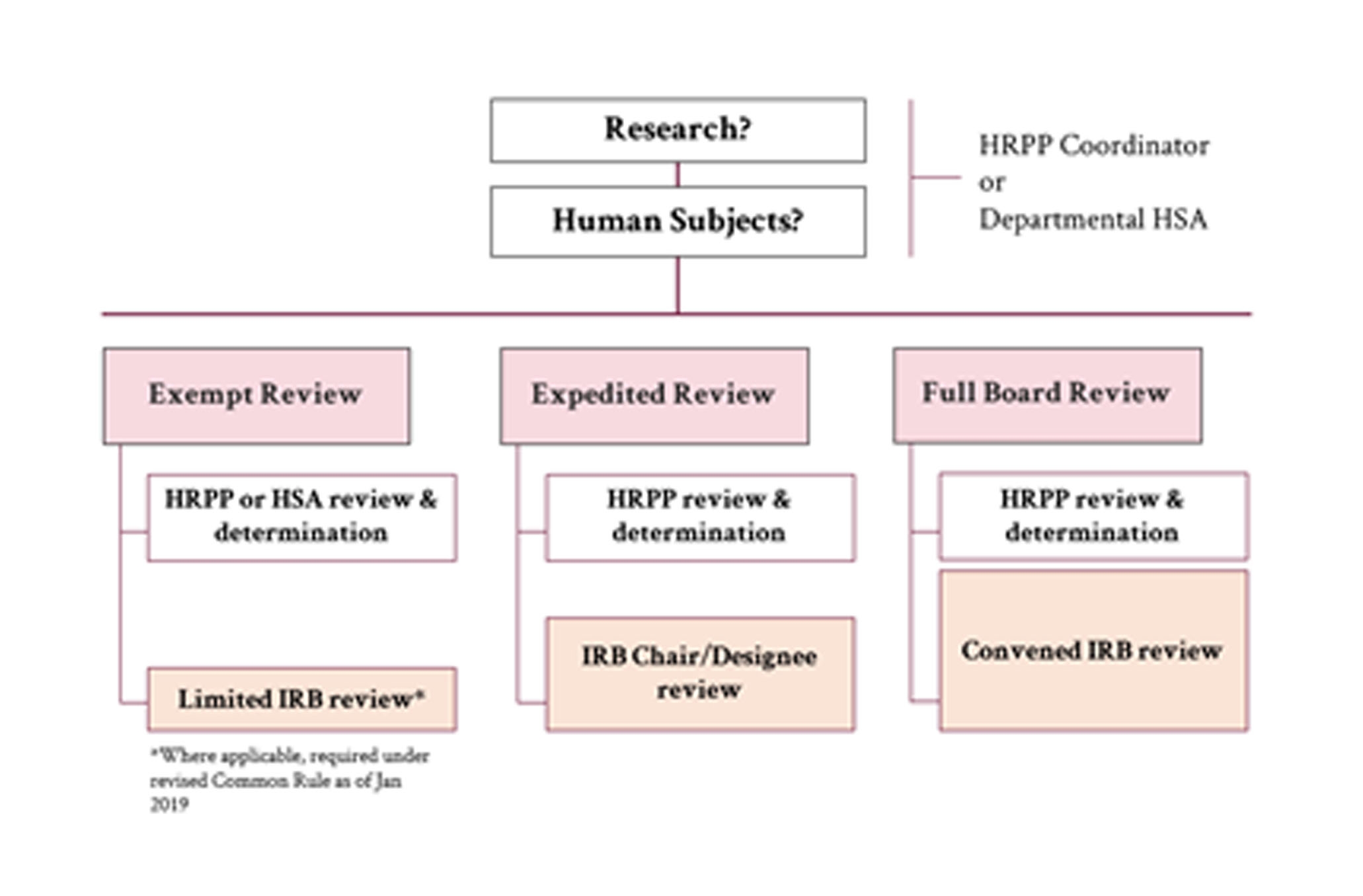
Exempt Review
Research can be approved as Exempt if it is no more than minimal risk, and fits one of the exempt review categories as defined by federal regulation 45 CFR 46. Exempt does not mean the protocol does not require HRPP or Institutional Review Board (IRB) review. Exempt determinations can only be made by a HRPP protocol coordinator.
Expedited Review
Research can be approved as Expedited if it is no more than minimal risk and fits in one of the federally designated expedited review categories. Expedited review does not necessarily mean that protocol reviews that fall into this category will be reviewed expeditiously. It means that it does not need to be reviewed by the convened IRB. Expedited reviews can only be done by an IRB member.
Full Board Review
Full Board studies are reviewed by the convened IRB. Research that involves greater than minimal risk and does not fit into an exempt or expedited category must be reviewed by the convened IRB. The Virginia Tech IRB meets once a month to review these studies.
Additional information on levels of review and federal regulations can be located on the Office for Human Research Protections website.
For any submission to the Human Research Protection program office, a researcher will need to have the overall responsibility for the oversight and conduct of the research. This person is the principal investigator (PI), faculty member, and/or an approved investigator. Each principal investigator will need to provide acknowledgement for each study through their Protocol Management profile. For students, this is typically a faculty advisor.
See the Office of Sponsored Programs’ Principal Investigator Eligibility Chart and Criteria for additional information.
For questions, email irb@vt.edu.
- Ensure that all documents that will be used with participants are uploaded. This includes but is not limited to: advertisements, social media posts, video transcripts, voice scripts, and emails (with the subject line).
- Check spelling and grammar
- Make sure all documents are final and do not include comments and edits
- Avoid common errors:
- Inconsistency: arefully read all documents before submitting; pay close attention to numbers, dates, and description of processes and procedures to make sure they are consistent across all documents
- Incomplete submission: Missing documents and questions not adequately addressed (use the protocol tips document)
- Submit: Once documents are uploaded, select “submit.” Once the protocol is submitted successfully it will no longer appear in working draft status within Protocol Management.
- Use available resources
- The resource section on the HRPP website has additional information to help with submissions.
- The worksheets and checklists posted are used by the HRPP Protocol Coordinators in conducting pre-reviews. These tools will help to determine the criteria that are used in reviewing submissions.
- Tips documents for protocols can be found on the Resources for Conducting Human Research page. Reviewing the tips documents will help avoid common mistakes.
- Use the current version of the consent form and information sheet, which are posted on the website. NOTE: It is important to get these from the website each time needed, rather than using a copy from an older protocol. These documents are updated regularly.
- For questions, there are weekly HRPP office hours or consultations available by sending an inquiry to irb@vt.edu.
- For questions about data collection systems, data storage requirements, or privacy and data security, please view the Privacy and Research Data Protection Program website.
For addtional questions, email irb@vt.edu.


