Research Administrative Programs and Oversight Committees
Virginia Tech provides essential administrative programs and oversight committees to researchers in an effort to enhance compliance, integrity, and safety of the research being conducted through the university. With these areas of support, researchers can focus more on their research activities while maintaining compliance.
Research Administrative Programs

Animal Care and Use Program
The Animal Care and Use Program is the administrative office that supports the Institutional Animal Care and Use Committee.

Animal Resources and Care Division
Virginia Tech’s Animal Resources and Care Division provides resources and expertise to support scientifically sound animal research and teaching activities using healthy animals in the appropriate environment.

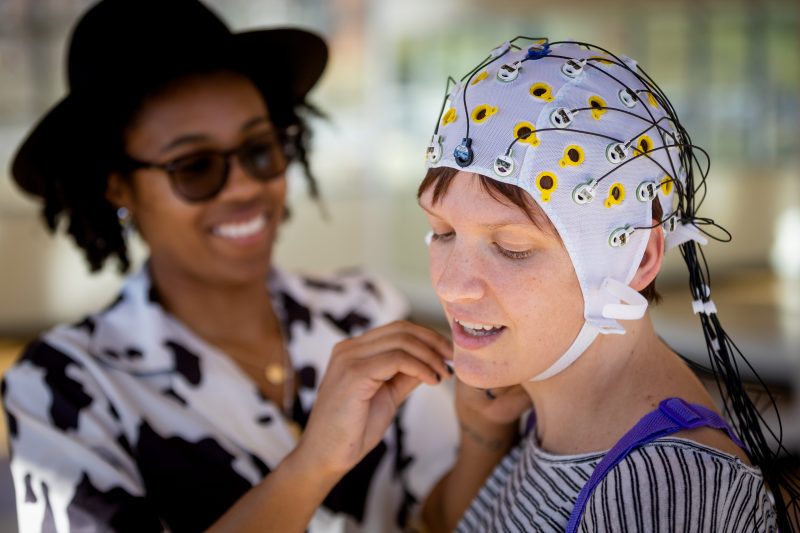
Human Research Protection Program
Virginia Tech’s Human Research Protections Program is designed to support researchers in meeting their ethical and regulatory responsibilities to human research participants.

Institutional Biosafetey Committee Program
The Institutional Biosafety Committee Program is the administrative office that supports the Institutional Biosafety Committee.

Office of Export and Secure Research Compliance
The Office of Export and Secure Research Compliance works with university departments to ensure compliance with regulations promulgated by U.S. Government agencies, including but not limited to the Department of State, Department of Commerce, and the Department of the Treasury.

Privacy and Research Data Protection Program
The Privacy and Research Data Protection Program assists Virginia Tech’s researchers with guidance needed to keep data secure and compliant with university, state, and federal regulations.

Research Conflict of Interest Program
The Research Conflict of Interest Program is responsible for assessing and implementing management strategies for investigator financial conflicts of interest. The program also administers the university's system for outside activity and financial interest disclosure.

Research Integrity and Consultation Program
The Research Integrity and Consultation Program develops and provides a comprehensive program of training experiences and learning opportunities to infuse ethics, and regulatory knowledge across the university. The program also provides a research ethics consultation service that helps researchers to identify, analyze and resolve complex questions that arise during the development, conduct, and dissemination of research.
Research Oversight Committees

Institutional Animal Care and Use Committee
The Institutional Animal Care and Use Committee ensures humane care for and the well-being of animals used in research and teaching at the university. As per federal law, the committee has the authority to approve, disapprove, or require modifications to be made to protocols to ensure regulatory compliance.

Institutional Biosafety Committee
The Institutional Biosafety Committees provides oversight of research that involves the use of biohazardous agents, including recombinant and/or synthetic nucleic acid molecules. The committee is charged with planning and implementation of the campus Biosafety Program with a purpose to ensure the health and safety of all personnel working with biohazardous agents.

Institutional Review Board
The Institutional Review Board protects research participants and reviews proposed research before it is implemented to ensure that it is scientifically valid, risks to participants are minimized, and that risks are reasonably relative to the expected benefits of the research.
Management Plan Advisory Committee
The Management Plan Advisory Committee is a standing committee of the university that makes recommendations to the program director regarding how financial conflicts of interest should be managed to ensure that sponsored research will be objective and free from bias to the extent possible.

Radiation Safety Committee
The Radiation Safety Committee is an operational committee responsible for regulating the safe use of all radioactive material and radiation-producing equipment at Virginia Tech.


